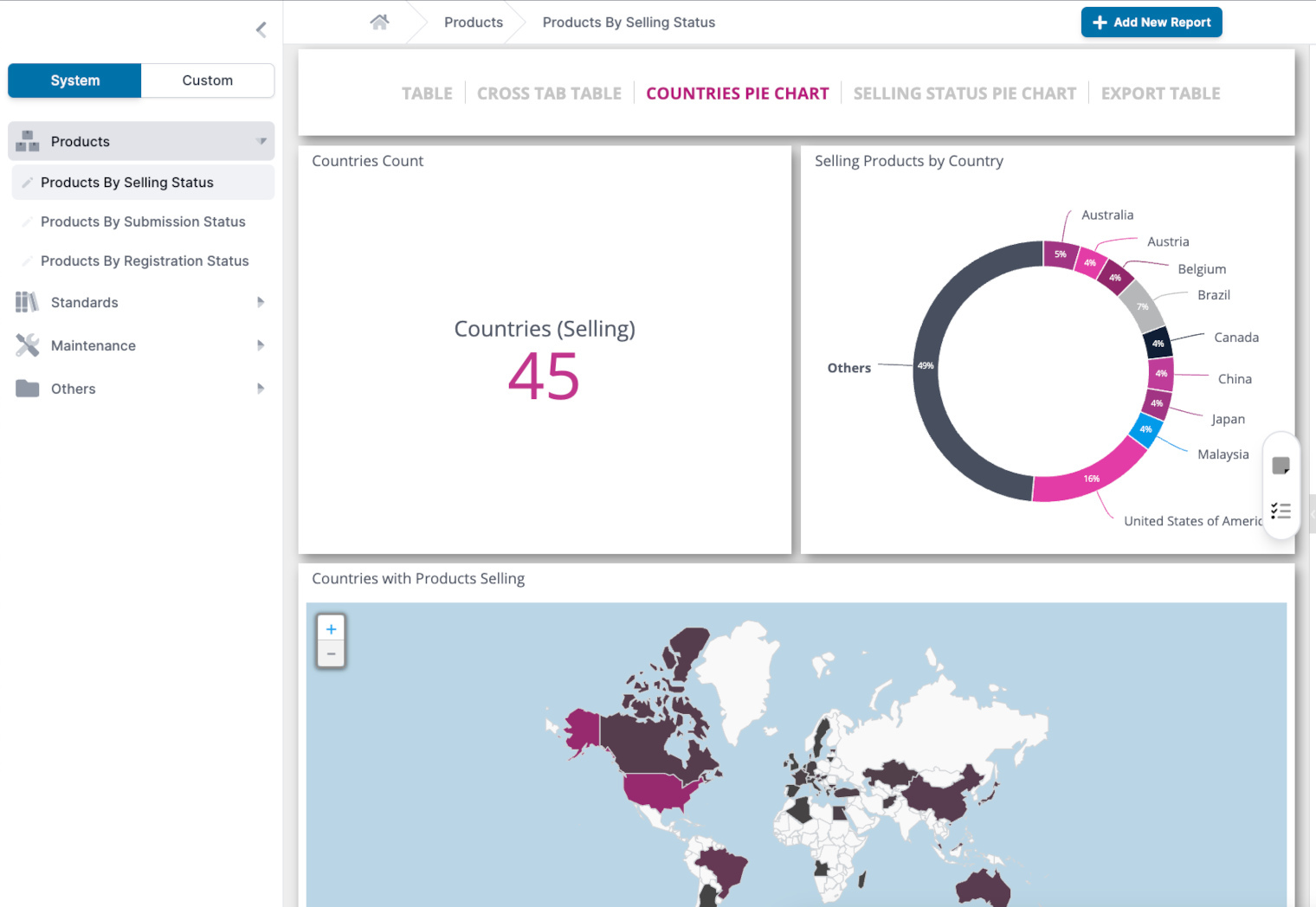
ANALYTICS
Comprehensive Analytics Dashboard
Centalized hub for global regulatory intelligence data. Create self-service application, product, and country reports to see exactly where your products can be sold and their current registration status

Revolutionising paperwork by making it paper-free


Why Choose Us?
Tailored solutions.
Seamless compliance.
Having worked with a diversity of tech enterprises, including giants like Amazon and American Express, we carry a rich experience to address your needs and develop a tailored software solution.
Automation and Efficiency
We'll keep track of when your certificates need renewing and take care of generating the necessary documentation for you.
Seamless Migration
We ensure a smooth transition with tailored solutions that adapt to your processes.
Local Understanding
With extensive knowledge of the widespread med-tech industry in India, our software is catered for precise local needs.
What's Included
Manage certifications and licenses with renewal alerts
Generate submission and technical documents for CE, ISO, and BIS marking.
Create workflows and manage approvals for traceability
Advanced life cycle management for all SKUs in one in-house database
Manage vendors and orders efficiently with integrated financial tracking
Generate e-catalogs and brochures for brand website effectively.
OUR MISSION
Making Medtech Paperless
Despite being a tech service export leader, we still depend on outdated paper trails. Our mission is to revolutionise medtech by making it entirely paper-free. We aim to automate compliance, empowering you to focus on what you do best - manufacturing.
Our software, proudly made in India, is specifically designed for Indian businesses. We won't impose rigid workflows; instead, we'll tailor our solutions to fit your unique requirements, helping you expand to new markets with ease and speed.
OUR FOUNDER
Software runs in our blood

SOFTWARE DEVELOPER
B.Tech, Computer Science
IIT Madras
Ex-Amazon | Ex-Amex
An IIT Madras graduate, with a proven track record of developing innovative platforms and scalable software. With experience at industry leaders like Amazon and dynamic startups such as Clockout, he brings a wealth of expertise across various tech domains.
Known for his versatility in software development, he has a diverse portfolio that includes IoT solutions, AI applications, and FinTech innovations. His ability to seamlessly navigate different technological landscapes makes him a valuable asset in driving forward-thinking projects.
SERVICES
Our amazing services
Effortlessly manage compliance with automation, AI, and real-time updates
Track relevant laws, regulations, guidance, and news in one place
Effortless SKU and UDI management, IFU generation, smart author system and connected workflow
Get notified of changing standards, expiring certificates and documents that impact your products
Connects easily with your ERP, OMS, PLM and QMS systems

ANALYTICS
Comprehensive Analytics Dashboard
Centalized hub for global regulatory intelligence data. Create self-service application, product, and country reports to see exactly where your products can be sold and their current registration status
Monitor certification statuses, renewal dates, and compliance metrics in real-time.
Analyze approval workflows and traceability to identify bottlenecks and optimize processes.
Our Platform
Please click on the items to know more about our platform
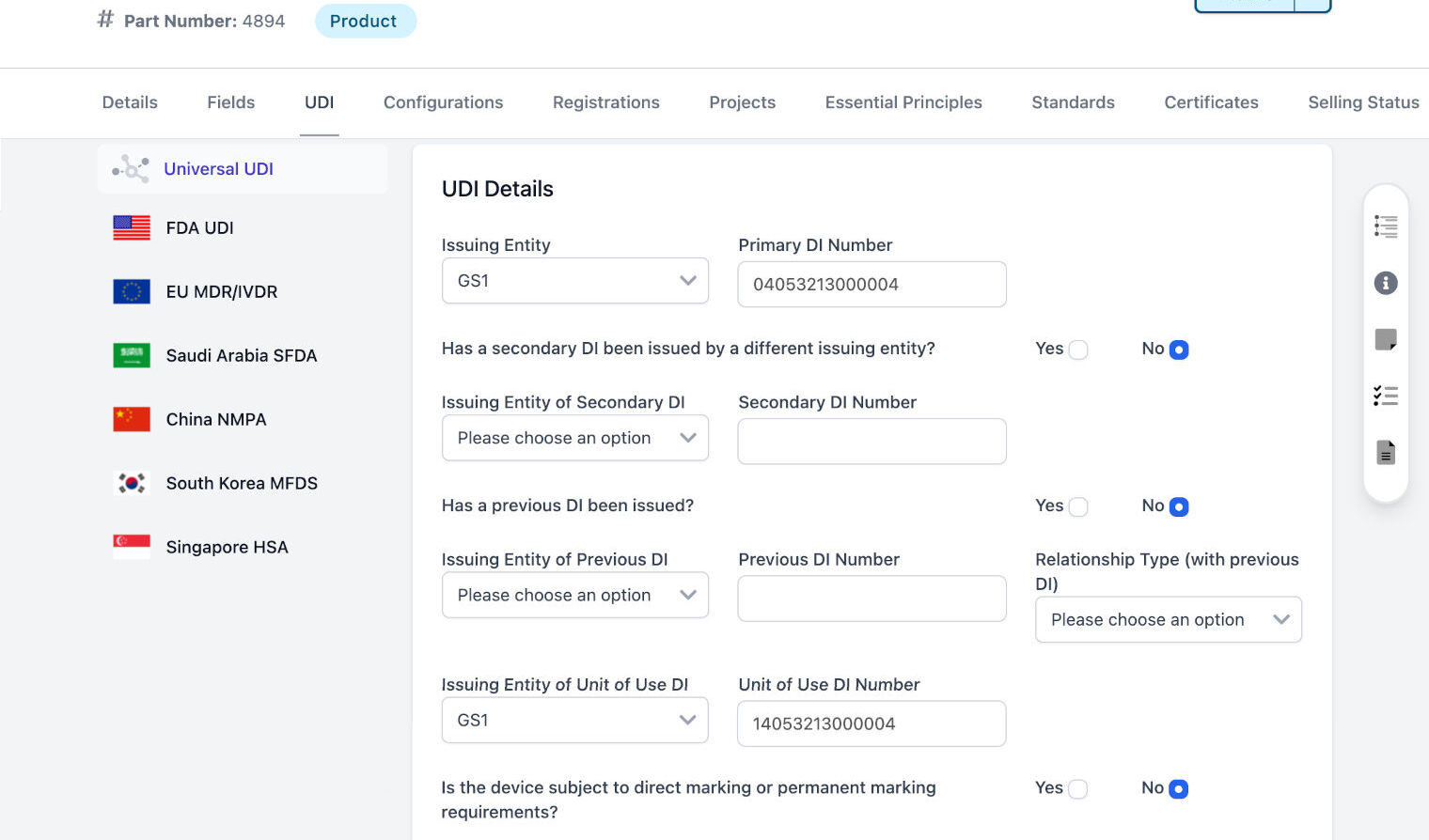
Collect, organize, and manage all regulatory information at the individual product level
Create self-service application, product, and country reports to see exactly where your products can be sold and their current registration status
Use regulatory intelligence and digital templates to guide regulatory strategy and streamline submissions
Streamline regulatory submissions with collaborative authoring, intelligent reusable content, and compliant PDF generation
Digitally collect and maintain evidence of compliance. Link to relevant standards and get notified of changes
Route approvals, assign tasks, and share notes across teams inside and outside of your organization
Get notified of changing standards, laws, regulations, and guidance documents that impact your products
Seamlessly transfer data and documents across all of your systems, and link selling status directly to your ERP/CRM